How Many Valence Electrons Does a Bromine Atom Have
- Covalent compounds are formed by sharing electrons between atoms - Most of the compounds that we come in contact with are covalent compounds - Covalent compounds contain covalent bonds - Nitrogen N2 is a covalent compound - Covalent compounds are formed by transferring electrons from one atom to another atom. The elements that have 1 2 or three electrons in the last shell donate the electrons in the last.
Chem4kids Com Bromine Orbital And Bonding Info
An s orbital is spherically symmetric around the nucleus of the atom like a hollow ball made of rather fluffy material with the nucleus at its centre.

. How many valence electrons of lithium ionLi have. In this case both the valence and valence electrons of lithium are one. The order of size is 1s 2s 3s.
Gold silver helium oxygen mercury hydrogen sodium nitrogen niobium neodymium chlorine carbon. Periodic Table of Elements 1. Periodic Table of Elements 3.
We know the details about this. As the energy levels increase the electrons are located further from the nucleus so the orbitals get bigger. After the electron configuration the last shell of the lithium atom has an electron.

How Many Valence Electrons Does Bromine Have
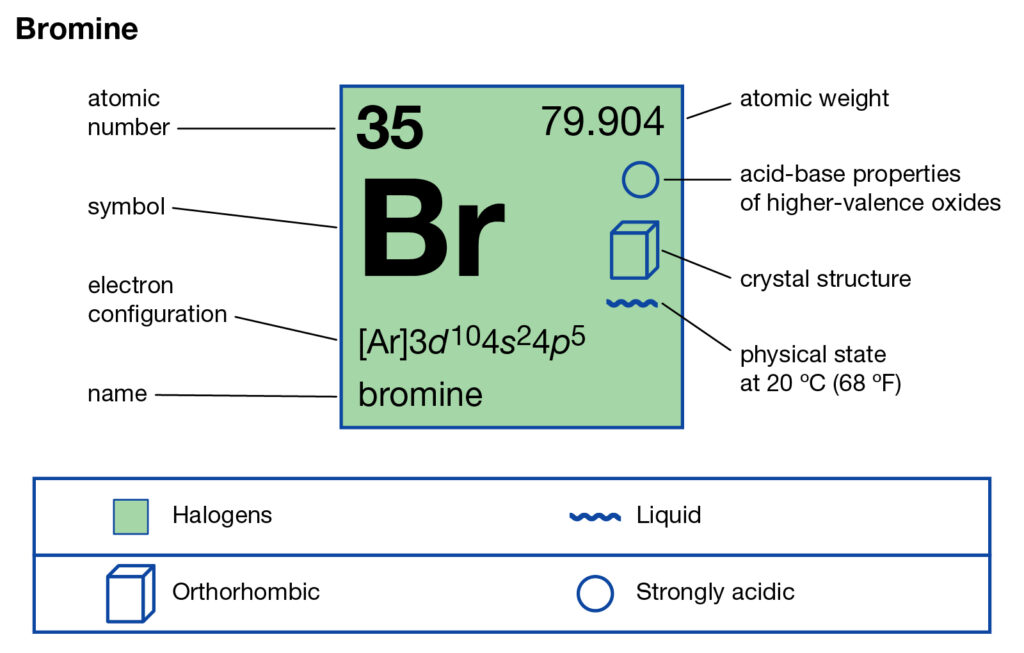
Bromine Valence Electrons Bromine Valency Br Dot Diagram

Comments
Post a Comment